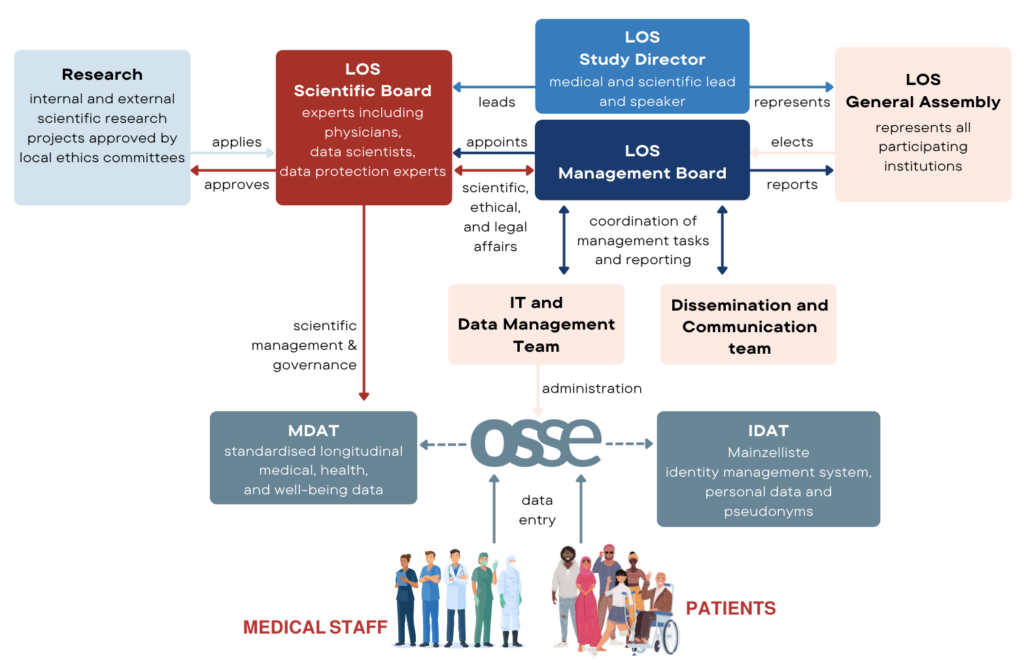
The governance of the LOS transfusion registry aims to ensure ethical and effective management through a structured and transparent framework. By fostering collaboration and involvement of researchers, healthcare professionals and policy makers, this framework enriches scientific potential and impact while maintaining high standards for data utilisation and participant confidentiality. We are strongly committed to inclusivity and ensure that different perspectives are represented and valued in our decision-making processes.
LOS Scientific Board
- Consists of physicians, data scientists, data protection experts, ethists, and other scientists.
- Monitors scientific integrity and compliance with data protection and ethical standards.
- Reports to the LOS Study Director, who is the medical and scientific lead.
LOS Management Board
- Elected by the LOS General Assembly and represents all participating organisations.
- Coordinates management activities and reporting to ensure smooth operation and alignment with the objectives of the study.
Dissemination and communication team
- Takes care of public relations and the distribution of information.
- Ensures that relevant information reaches participants, professional and patient organisations, the scientific community and the public.
IT and data management team
- Responsible for the technical aspects of data processing and security.
- Ensures compliance with the General Data Protection Regulation and other relevant regulations and adheres to the highest data protection standards.
By establishing this comprehensive governance structure, the LOS Transfusion Registry ensures ethical and efficient operations, contributing to safer transfusion practices and broader public health outreach.