The Clock Is Ticking on EU Blood Management
Achieving SoHO compliance - start today!
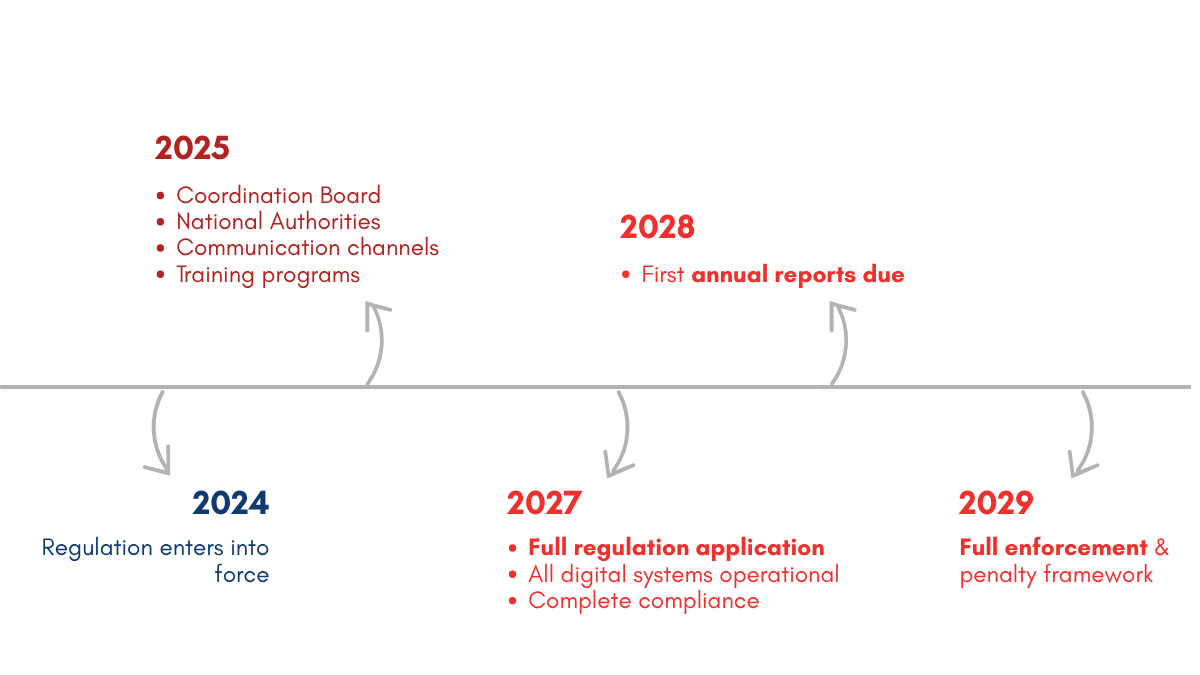
In August 2027, everything changes. The EU SoHO Regulation 2024/1938 will transform how every drop of blood is tracked, reported, and shared across Europe. This is not just another regulatory update – it is a complete digital revolution in blood management. Right now, across the EU, we transfuse over 17 million units of blood to more than 3 million patients annually. Yet our systems remain fragmented, with 27 different approaches creating barriers when patients need blood most. The new regulation demands one standard for every patient and blood donor in Europe.
What this means for your institution
By August 2027, your blood bank will not just need to be digital—it must be connected to the EU-SoHO platform, tracking every unit from donor to recipient and storing the data for 30 years. Annual reports that once took months must be submitted by June 30th each year. When adverse events occur, you will have just 24 hours to report them. And when critical shortages hit, your inventory levels must be visible across EU borders in real time. This is not about compliance checkboxes. It is about joining a European blood network that can respond to crises, eliminate waste, and save lives. we have less than three years until the full compliance deadline, otherwise risking sanctions as early as in 2029.
Start your compliance journey
Why wait when you can lead?
The LOS Registry is ethically approved (DRKS00034405), open-source, and ready for immediate deployment. It is been tested in real hospital environments, refined through hundreds of transfusions, and expandable to meet EU SoHO compliance. Every hospital will need a digital blood management system. The question is whether you will lead your region in compliance or follow others who started today.
The EU is not just mandating change – it is offering an opportunity to revolutionize blood safety. Countries and hospitals that move first will shape how this transformation unfolds. They will access EU funding for digital health initiatives. They will attract partnerships and research opportunities. Most importantly, they will provide better, safer care for their patients.
The team behind the LOS Registry has spent years preparing. As anesthesiologists and intensive care medical professionals, we understand the complexity of blood management, the pressure of regulatory compliance, and the importance of getting this right. We are looking forward to work together with other hospitals, regional and national policymakers and governments and the EU on the path to a sustainable digital transformation of blood donations and transfusions. Contact us today!
recorded LOS registry data
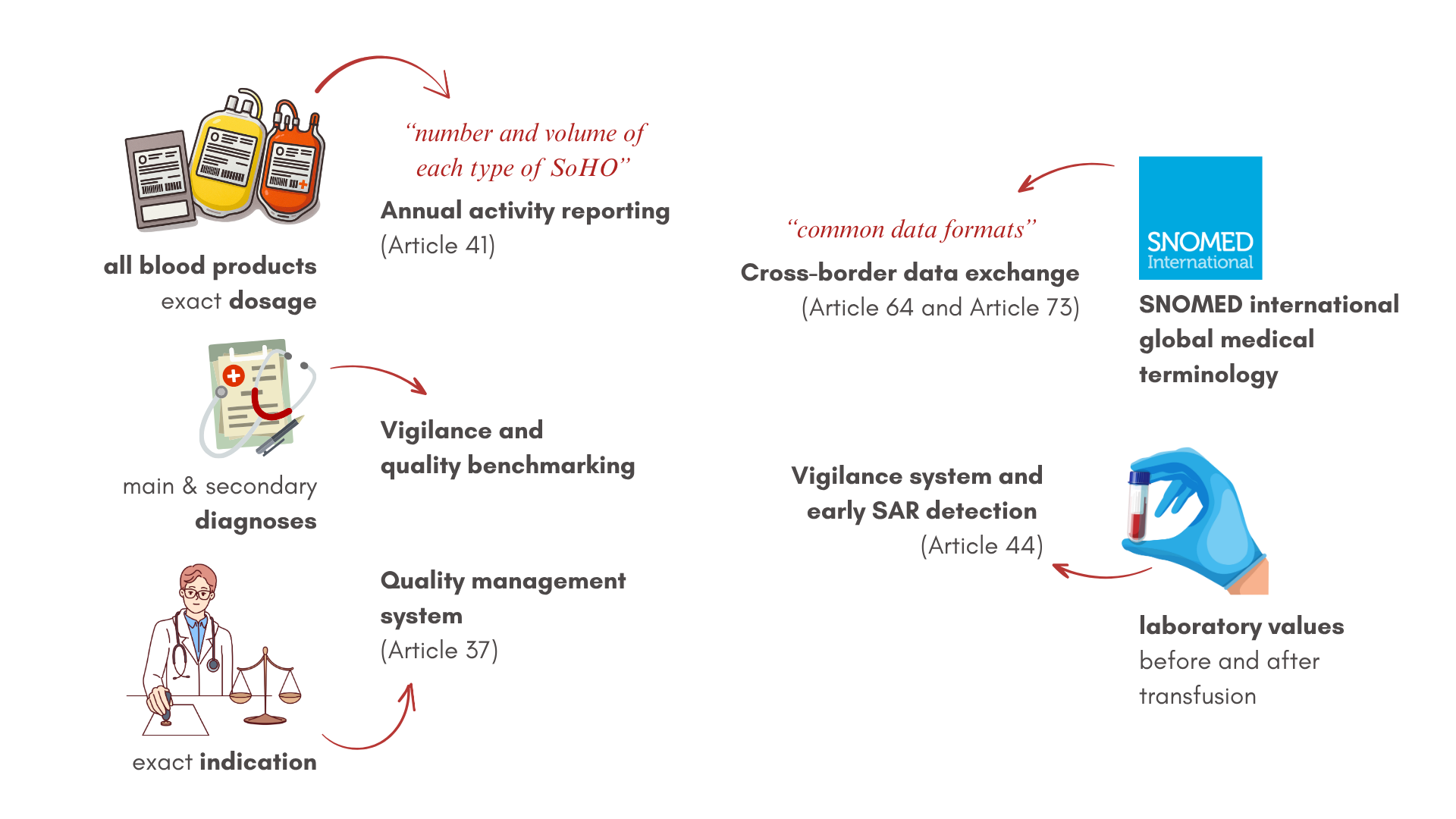
Direct relevance to numerous SoHO Articles
A Comprehensive Framework for Substances of Human Origin
Understanding the EU SoHO regulation 2024/1938
The Regulation (EU) 2024/1938 on substances of human origin (SoHO) represents the most significant transformation in European blood and tissue management in over two decades. Adopted by the European Parliament and Council on June 13, 2024, this regulation establishes unified quality and safety standards for all substances of human origin intended for human application across the European Union.
This comprehensive framework replaces the previous Blood Directive (2002/98/EC) and Tissues and Cells Directive (2004/23/EC), expanding its scope to encompass all human-derived therapeutic substances while introducing mandatory digital infrastructure requirements that will fundamentally reshape how healthcare institutions manage these critical resources.
Scope and application
The SoHO regulation specifically excludes solid organs for transplantation and human milk intended for a mother’s own child, which remain governed by separate legislative frameworks. It applies to all substances derived from the human body intended for clinical application, including:
- Blood and blood components (red blood cells, plasma, platelets)
- Human tissues and cells, including hematopoietic stem cells
- Reproductive cells (gametes) and embryos
- Human milk for medical purposes
- Fecal microbiota for transplantation
- Any other therapeutic substances of human origin
Core objectives of the regulation
The SoHO Regulation pursues four fundamental objectives that address longstanding challenges in European healthcare:
1. Harmonization across member states
The regulation eliminates the fragmented landscape of numerous different national implementations, establishing uniform standards that facilitate seamless cooperation between EU healthcare systems. This harmonization ensures that a patient in any member state receives substances meeting identical safety and quality standards.
2. Enhanced patient safety
Through mandatory traceability systems and real-time vigilance reporting, the regulation creates an unprecedented level of oversight for substance safety. Each unit must be traceable from donor to recipient for at least 30 years to enable a rapid response to emerging safety concerns and demonstrate a commitment to long-term safety – as does our innovative registry study.
3. Supply security and crisis response
The regulation mandates national emergency preparedness plans and cross-border cooperation mechanisms. During critical shortages or public health emergencies, member states must share inventory data and coordinate distribution to ensure no patient lacks access to life-saving substances.
4. Innovation and future-readiness
By establishing technology-neutral requirements, the regulation accommodates emerging therapies while maintaining rigorous safety standards. This forward-looking approach ensures the framework remains relevant as medical science advances.
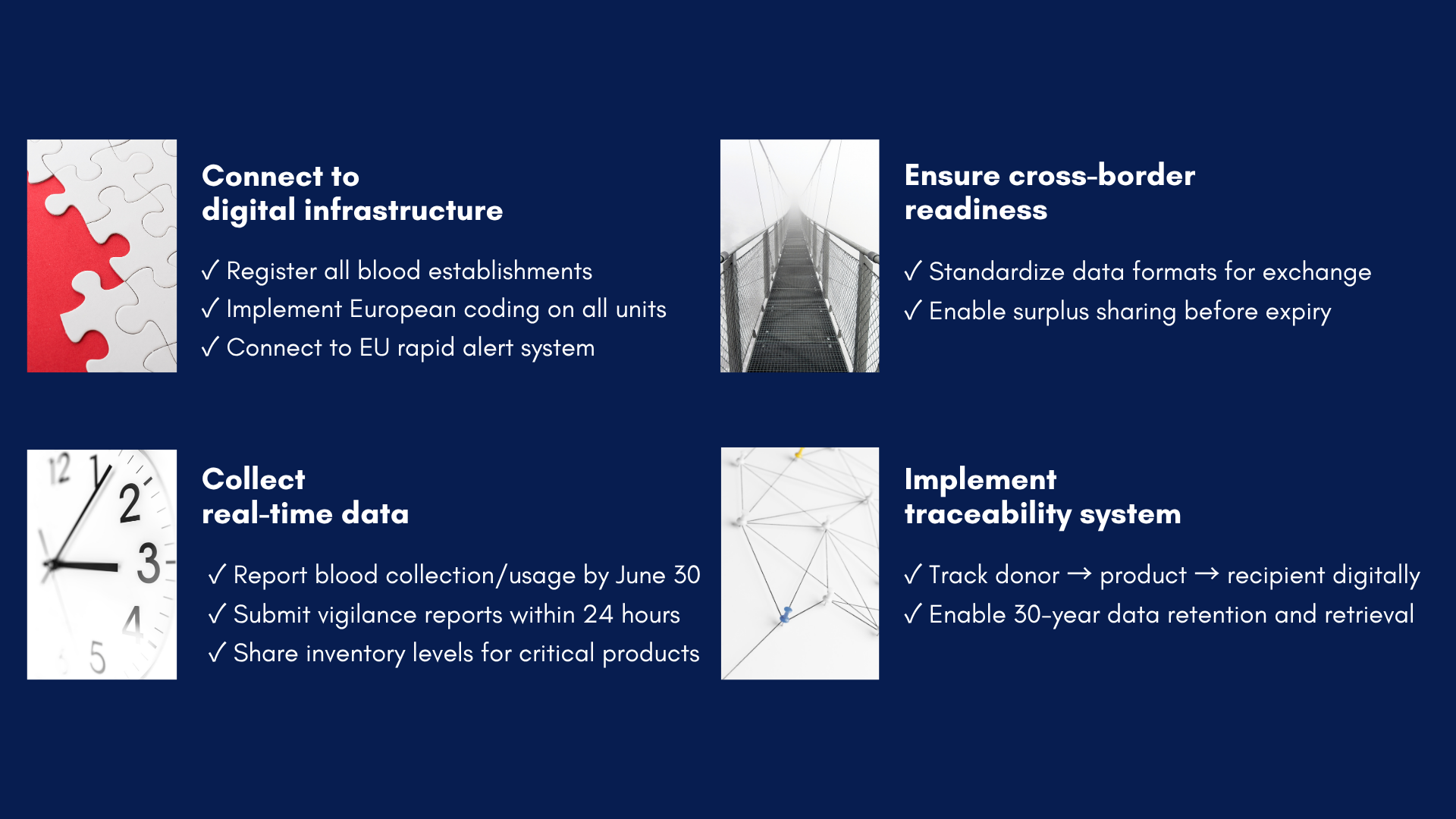
The four pillars of compliance
Connect to digital infrastructure
Healthcare institutions must transition from paper-based systems to integrated digital platforms. This transformation requires registering all establishments in the EU-SoHO platform, implementing standardized European coding systems for SoHO units, and establishing real-time connections to the rapid alert network. These digital foundations enable the instantaneous communication essential for modern healthcare delivery.
Collect real-time data
The regulation mandates comprehensive data collection and reporting capabilities. Institutions must submit detailed annual activity reports by June 30 of each following year, report serious adverse reactions and events within 24 hours of detection, and maintain current inventory levels for critical substances. This continuous data flow enables evidence-based decision-making and quality improvement across the European blood supply network.
Ensure cross-border readiness
Recognizing that patient needs transcend national boundaries, the regulation requires standardized data formats that enable seamless information exchange. Institutions must implement systems enabling surplus sharing before expiration, preventing waste while addressing shortages. This interconnected approach transforms isolated national systems into a unified European network.
Implement traceability system
Perhaps the most technically demanding requirement involves establishing complete traceability from donor through processing to final recipient. This system must maintain records for 30 years in a format enabling rapid retrieval and analysis. Such comprehensive tracking enables lookback investigations when safety concerns emerge and provides the epidemiological data necessary for continuous improvement.
Preparing for success
The SoHO Regulation forms part of the European Health Union initiative, complementing other digital health transformations including the European Health Data Space. By establishing interoperable systems for substances of human origin, the regulation creates foundational infrastructure for broader healthcare integration.
This regulatory framework positions Europe as a global leader in blood and tissue safety, potentially influencing international standards. Countries outside the EU observe these developments closely, recognizing that European requirements often presage global trends.
Success under the SoHO Regulation requires immediate action. Institutions beginning implementation now benefit from longer adaptation periods, opportunity to influence emerging technical standards, and access to early-adopter support programs. Conversely, delayed action risks compressed implementation timelines, limited vendor capacity as deadlines approach, and potential non-compliance penalties.
The regulation represents both a challenge and an opportunity. While compliance demands significant effort, the resulting infrastructure creates lasting benefits through improved patient safety, operational efficiency, and participation in a revolutionary European healthcare network.







Subscribe
Get latest updates on the LOS Registry Study and advancements in transfusion medicine
Registry Study for Research of Blood Donor and Recipient Long-Term Outcomes
The latest news, articles, and resources on transfusion medicine – follow our deputy study director, Dr Jan Kloka, on LinkedIn.
Address
Universitätsklinikum
Frankfurt am Main
KAIS – Haus 13a
Theodor-Stern-Kai 7
60596 Frankfurt am Main
Contact
© Copyright 2024 by transfusionregistry.org